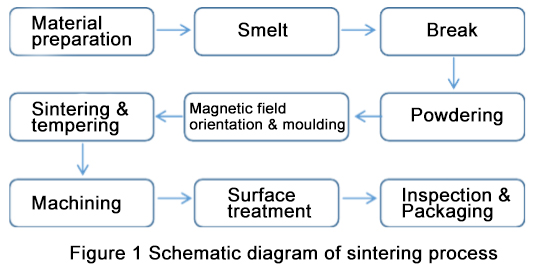
With the continuous development of science, sintering methods of zirconia ceramics are continuously introduced.
Electric field sintering
Electric field sintering refers to the sintering of the ceramic body under the action of a DC electric field. Some high-curie-point ferroelectric ceramics, such as lithium niobate ceramics, apply a DC field to both ends of the green body at their sintering temperature. After cooling to a temperature below the Curie point (Te-1210 ℃) and removing the electric field, you can obtain Piezoelectric ceramic samples.
Ultrahigh pressure sintering
Ultra-high pressure sintering is sintering at a pressure of several hundred thousand atmospheres or more. Its characteristics are that it cannot only make the material reach high density quickly, and have fine grains (less than 1um), but also change the crystal structure and even the atomic and electronic states, so that the material cannot be reached under the usual sintering or hot-pressing sintering process Performance, and can synthesize new artificial minerals. This process is relatively complicated and requires higher mold materials, vacuum sealing technology, and fineness and purity of raw materials.
Activated sintering
The principle of activated sintering is to use some physical or chemical methods to make the atoms or molecules of the reactants in a high-energy state before or during sintering. With the instability of this high-energy state, it is easy to release energy Low energy state. The physical methods used in activated sintering include electric field sintering, magnetic field sintering, sintering under the action of ultrasound or radiation, and so on; the chemical methods used are: chemical reactions based on redox reactions, dissociation of oxides, halides, and hydroxides, and atmospheric sintering. Activated sintering has the advantages of reducing the sintering temperature, shortening the sintering time, and improving the sintering effect.
For some ceramic materials, activated sintering is another effective texturing technique. There is also the use of substances in the phase change, dehydration and other decomposition processes, the atom or ion bond is destroyed, making it in an unstable active state. For example, increase the specific surface area; add substances that can generate new erbium molecules during the sintering process; add substances that can promote the sintering material to form a solid solution; increase the lattice defect substances, all of which are activated sintering. In addition, activated sintering also includes adding a small number of substances that can form an active liquid phase, promote the vitrification of materials, appropriately reduce the viscosity of the liquid phase, wet the solid phase, and promote solid-phase dissolution and recrystallization.
Activated hot sintering
Activated hot-pressing sintering is a new process developed on the basis of activated sintering. It utilizes an activated state with higher energy during the decomposition reaction or phase change of the reactants to perform hot-pressing treatment, which can be performed at lower temperature and lower pressure. It is a high-efficiency hot pressing technology to obtain high-density ceramic materials in a short time. For example, barium titanate, lead zirconium titanate, ferrite, and other electronic ceramics are made by hot pressing by the decomposition reaction of hydroxide and oxide; high-density beryllium oxide, thorium oxide and uranium oxide ceramics were prepared by hot pressing of carbonate decomposition reaction; high-density alumina ceramics are made by hot pressing during phase transition of some materials.
Stanford Advanced Materials (SAM) is a global supplier of pure metals, alloys, ceramics, minerals, and rare earth materials since 1994. Headquartered in Lake Forest, California, SAM specializes in providing high-purity chemicals (up to 99.99999%) for research institutes and technical grade materials for advanced industries, such as pharmaceutical, capacitor, metallurgy, semiconductor, and aviation. Please visit https://www.samaterials.com/ for more information.