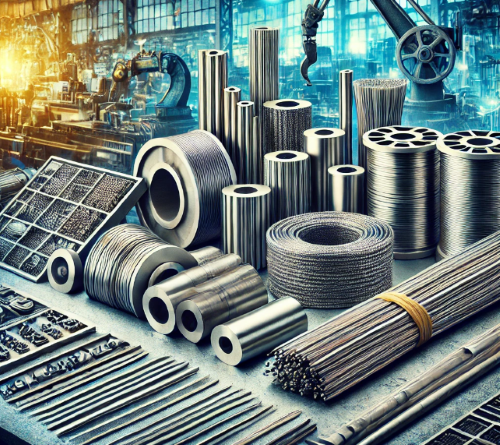
Zirconium wire is a highly versatile material known for its exceptional corrosion resistance, high melting point, and strength at elevated temperatures. These properties make it essential in industries that demand performance under extreme conditions, such as nuclear, chemical processing, aerospace, and medical applications.

Types of Zirconium Wire
- Pure Zirconium Wire (Zr 702)
Pure Zr wire (Zr 702) is prized for its outstanding corrosion resistance, particularly in acidic environments. It is commonly used in nuclear reactors, chemical processing, and aerospace applications where material durability and resistance to radiation are critical. - Zirconium Alloy Wire (Zr 705, Zr 704, Zr 703)
Zr alloy wire is produced by adding elements like tin (Sn), iron (Fe), or chromium (Cr) to enhance specific properties such as heat resistance, strength, and weldability. For example, Zr 705 offers excellent resistance to high-temperature oxidation, while other alloys like Zr 702 enhance mechanical properties for specialized use cases. - High-Purity Zirconium Wire
For critical applications that require minimal contamination, high-purity Zr wire ensures optimal performance in medical implants, electronic components, and other sensitive fields. - Zirconium Wire Coated with Other Materials
To further enhance performance, Zr wire can be coated with materials like nickel, silver, or copper. These coatings improve properties like electrical conductivity and resistance to extreme wear, making it suitable for a variety of advanced applications.

Here’s a summary table based on the types of zirconium wire:
| Types | Key Features | Common Uses |
| Pure Zirconium Wire (Zr 702) | Excellent corrosion resistance, especially in acidic environments. | Nuclear reactors, chemical processing, aerospace |
| Zirconium Alloy Wire (Zr 705, Zr 704, Zr 703) | Enhanced heat resistance, strength, and weldability with added elements (Sn, Fe, Cr). | High-temperature applications, mechanical stress applications, aerospace |
| High-Purity Zirconium Wire | Minimal contamination, optimal performance in sensitive environments. | Medical implants, electronics, critical components |
| Zirconium Wire Coated with Other Materials | Coatings of nickel, silver, or copper to improve conductivity and wear resistance. | Advanced electronics, high-wear environments, electrical applications |
Uses of Zirconium Wire
- Nuclear Industry
Zr wire, especially Zr 702, is widely used in nuclear reactors for fuel rod cladding. Its ability to resist radiation and high temperatures, combined with low neutron absorption, makes it ideal for containing nuclear fuel. - Chemical Processing
Zr wire is critical in the chemical industry for components exposed to aggressive chemicals. It is commonly used in heat exchangers, reactors, and piping systems, where its resistance to corrosion from acids and alkalis is essential. - Aerospace
In aerospace, zirconium wire is used for components exposed to extreme temperatures, such as heat shields, rocket nozzles, and turbine blades, due to its excellent thermal stability and strength. - Medical Applications
Due to its biocompatibility and corrosion resistance, Zr wire is used in medical implants, surgical instruments, and dental products, ensuring durability and non-reactivity within the human body. - Electrical & Electronics
Zr wire is utilized in high-temperature and high-corrosion environments for thermocouples, heating elements, and electrodes, where other materials would fail due to extreme conditions. - Automotive Industry
In the automotive industry, Zr wire plays a vital role in catalytic converters, exhaust systems, and fuel cells. Its high resistance to both heat and corrosive gases makes it ideal for harsh automotive environments. - Precision Welding
Zirconium wire is also used for precision welding in applications like aerospace and nuclear industries, where it is essential to withstand extreme temperatures and provide reliable, durable joints.
Why Choose ARM’s Zirconium Wire?
- Exceptional Corrosion Resistance: Ideal for use in harsh chemical and nuclear environments.
- High Strength & Durability: Withstands high temperatures and mechanical stress.
- Custom Solutions: Including Niobium Zirconium Alloy Wire for specialized applications.
- Precision Manufacturing: Offering consistent quality and reliable performance.
Contact ARM Today
At Advanced Refractory Metals (ARM), we supply high-quality zirconium wire, including pure zirconium wire (Zr 702), zirconium alloy wire, and high-purity zirconium wire. Whether you need straight wire, spool wire, or custom lengths, we have the right solution for your needs. Contact us today to learn more or request a quote!










 [1]
[1]