Zirconium is a metal material with superior physical and chemical characteristics. It is used in a variety of industrial, scientific, and technological applications. The following is an analysis of the uses and prospects of zirconium from different angles.
Zirconium Used in Nuclear Energy
Zirconium is one of the essential elements in the realm of nuclear energy due to its physical characteristics. Fuel rods and structural components for nuclear reactors can be manufactured with zirconium alloys. The high melting point, corrosion resistance, high strength, and high-temperature stability of zirconium alloys make them ideal materials for producing nuclear reactor fuel rods. Statistics show that every year, roughly 50 tons of zirconium alloys are used in the production of nuclear reactors worldwide.

Zirconium Used in Aerospace Industry
Zirconium is frequently utilized in the aerospace industry due to its superior mechanical qualities and strong temperature endurance. Zirconium alloys can be used to create components for aero engines such as blades, nozzles, and combustion chambers. Zirconium alloys can be utilized for a variety of components, including spacecraft hulls, turbines, and combustion chambers. They have exceptional qualities that can enhance spaceship performance, including lightweight, high strength, and high-temperature durability.
Zirconium Use in Medical Field
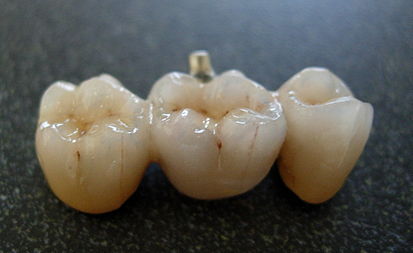
Zirconium is used extensively in the medical industry. Drugs can be radiolabeled using the zirconium isotope zirconium-89 for the detection and management of certain malignant disorders. Zirconium alloys have high strength, strong biocompatibility, and corrosion resistance, which can increase long-term durability and biological compatibility, and they can also be utilized to make artificial joints, dental implants, and other biomedical materials.
Zirconium Used in Chemical Industry
The chemical sector additionally employs extensive use of zirconium. Zirconium compounds are used in a variety of industries, including oxidants, antiseptics, catalyst supports, and catalysts. Because zirconium alloys offer great corrosion resistance, high-temperature stability, and long-term use in hostile chemical environments, they can also be utilized to make reactors, heat exchangers, reactors, and other equipment.
Zirconium Used in Electronics
Zirconium is also widely used in the field of electronics. Zirconium alloys and zirconates can both be used to create capacitors and battery electrodes, respectively. The primary areas of zirconium used in the electronics sector are nanotechnology and high-temperature superconducting materials. Zirconium can be used as an addition to boost the superconducting temperature and current density of high-temperature superconducting materials. Zirconium is also frequently utilized in nanotechnology and is capable of producing nanotubes, nanocrystals, and nanomaterials.
Zirconium Used in Metal Surface Coating
To stop corrosion and increase the hardness of metal surfaces, zirconium can be utilized in the production of surface coatings. Zirconium alloys can also be used to create metal coatings that are resistant to corrosion at high temperatures and have great corrosion resistance. Zirconium alloys are also perfect for producing drill bits, saw blades, and other tool materials due to their wear durability, and corrosion resistance.
Related reading: Where Zirconium is Used?
Conclusion
To sum up, zirconium has significant uses in the sectors of nuclear energy, aircraft, medical treatment, the chemical industry, electronics, and metal surface coating due to its exceptional physical and chemical qualities. The sustainable development of zirconium and the creation and use of ecologically friendly materials will also become popular trends as people’s awareness of environmental protection rises, further broadening the material’s potential uses.